Chasing a Cure: Genetics Researchers Pave the Way for Rare Hereditary Diseases
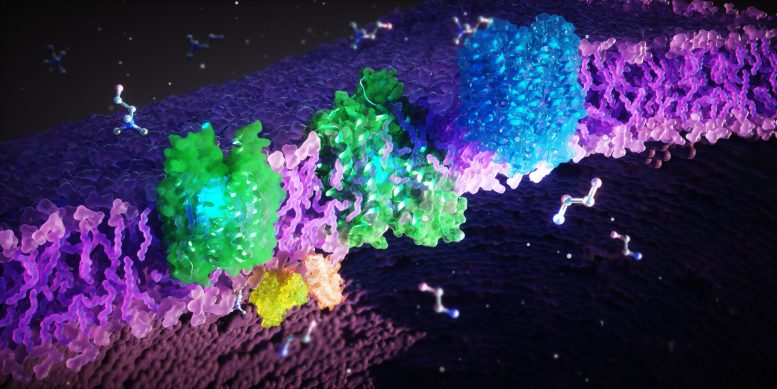
A group of international researchers has successfully identified the various structures and important functions of FLVCR1 and FLVCR2 proteins, which help to transport crucial molecules that affect cellular health. This new research sheds light on rare genetic disorders linked to these proteins and potentially paves the path for the development of new therapies. FLVCR proteins are present in the cellular membrane and their role involves the transportation of cellular building blocks like ethanolamine and choline across the membrane.
It's well-known that any defects in proteins, such as FLVCR1 and FLVCR2, can result in rare hereditary diseases in humans that can cause neurological, sensory, and motor disorders. However, the physiological functioning and biochemical mechanisms responsible behind such disorders weren't known until now.
Researchers from the USA, Singapore, and Frankfurt am Main collaborated in an interdisciplinary team to determine the 3D structures and cellular functions of FLVCR proteins. What they found was these proteins help to transport cellular building blocks called choline and ethanolamine. This discovery provides valuable insights into the cause of rare diseases and can contribute to the development of new treatments.
In reality, the process of finding correct diagnosis and suitable treatments for patients with unique or puzzling symptoms, as depicted in medical TV shows like Scrubs or Dr. House, often takes years. In many of these cases, no effective medication exists and therapeutic options are minimal.
Nearly 6-8% of the global population suffer from a rare disease. This amounts to around 500 million people. Even though each of the over 7000 different diseases only affects about one in every 2000 people, since they are so rare, scientific and medical understanding about them are limited. There are very few experts in the world, and also social awareness of these diseases are minimal.
An international team of researchers led by Schara Safarian, project group leader at the Max Planck Institute of Biophysics as well as independent group leader at the Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, and the Institute of Clinical Pharmacology at Goethe University Frankfurt, carefully studied two proteins known as FLVCR1 and FLVCR2. These proteins are known to play crucial roles in numerous rare hereditary diseases. Their findings were published in the prestigious scientific journal, Nature.
A gene mutation-caused malfunction of FLVCR1 or FLVCR2 can result in rare diseases. Some of these can lead to severe sensory, mobility, and visual disorders including Fowler’s Syndrome or sensory and autonomic neuropathies, and posterior column ataxia with retinitis pigmentosa. The latter can lead to a complete loss of pain sensation. Schara Safarian stresses that to understand such diseases and develop therapies, it is necessary to know the molecular structure and the function they perform in healthy cells.
Researchers discovered that these two proteins help to transport the molecules ethanolamine and choline across our cell membranes. These molecules are necessary for several major bodily functions. They help in the growth, stability and regeneration of our cells, for example in our internal organs, muscles, and brain. Choline particularly plays a crucial role in fat metabolism and detoxification process by the liver. It is also required by our body to produce the neurotransmitter acetylcholine which controls the organs and is vital for our nervous system. Hence, the malfunctioning of these proteins can potentially cause severe muscular and neurological disorders.
The researchers employed an array of methods to investigate these proteins, including microscopic, biochemical, and computer-assisted methods. The proteins were frozen and then studied under an electron microscope. An electron beam penetrates the frozen sample and the interaction of the electrons with the material forms an image. Multiple images are taken and processed which are then combined computationally to determine high-resolution 3D structures of proteins. By doing so, they cracked the structures of both FLVCR1 and FLVCR2 and observed their changes in the presence of ethanolamine and choline. Computer simulations confirmed how these proteins interact with ethanolamine and choline, and dynamically alter their structure to facilitate nutrient transport.
Safarian summarizes: “Our findings pave the way for understanding the development and progression of rare diseases associated with the FLVCR proteins. In the future, patients may be able to benefit from new therapies that restore their life quality.”
Reference: “Molecular mechanism of choline and ethanolamine transport in humans” by Keiken Ri, Tsai-Hsuan Weng, Ainara Claveras Cabezudo, Wiebke Jösting, Yu Zhang, Andre Bazzone, Nancy C. P. Leong, Sonja Welsch, Raymond T. Doty, Gonca Gursu, Tiffany Jia Ying Lim, Sarah Luise Schmidt, Janis L. Abkowitz, Gerhard Hummer, Di Wu, Long N. Nguyen and Schara Safarian, 22 May 2024, Nature. DOI: 10.1038/s41586-024-07444-7




