FDA Issues Warning on Overdosing from Unapproved Weight Loss Medications
The Food and Drug Administration (FDA) is warning people of the dangers of using compounded semaglutide, saying that people are overdosing on the products.
Dosage errors have led to symptoms such as nausea, vomiting, abdominal pain, and fainting, the agency said in a July 26 statement.
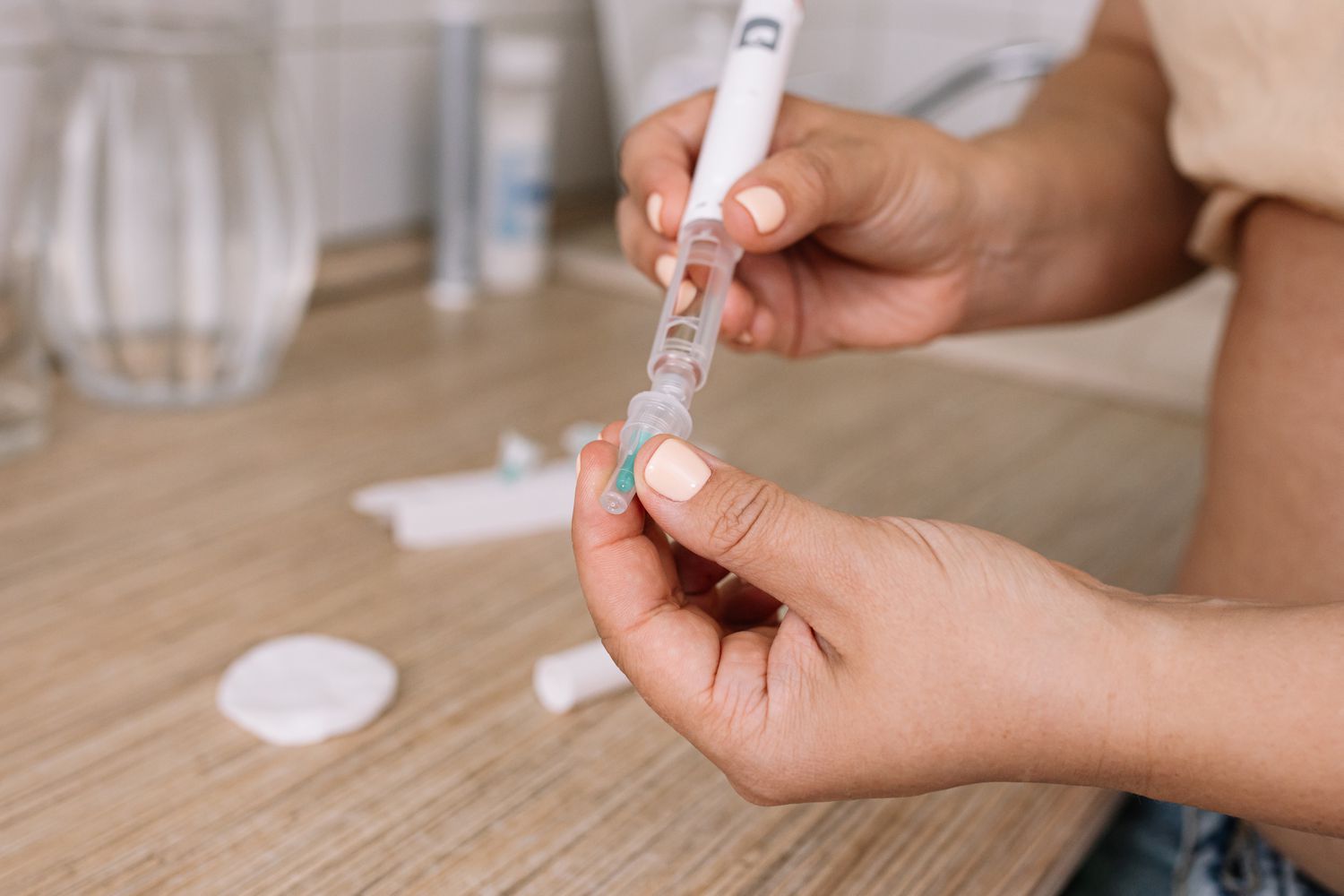
Semaglutide has grown in popularity for its weight loss management benefits. FDA-approved semaglutide products Ozempic and Wegovy are available as single-dose prefilled pens that call for weekly injections. Meanwhile, compounded semaglutide, which is not FDA-approved, often requires people to measure and draw the medication from a vial before self-administering an injection.
In the statement, the agency said that errors have resulted from patients making measuring mistakes and healthcare providers miscalculating appropriate dosages.
“Many of the patients who received vials of compounded semaglutide lacked experience with self-injections, according to the adverse event reports,” the FDA said. “Unfamiliarity with withdrawing medication from a vial into a syringe and coupled with confusion between different units of measurement (e.g., milliliters, milligrams, and ‘units’) may have contributed to dosing errors.”
Semaglutide belongs to a class of medications known as glucagon-like peptide-1 (GLP-1) receptor agonists. In addition to the prescription drugs Wegovy and Ozempic, the FDA has also approved Rybelus, an oral tablet that is also only available by prescription.
When there’s a shortage of FDA-approved drugs or an approved drug cannot meet a person’s medical needs, facilities have the legal authority to offer compounded drugs to fill that gap. The drugs include ingredients mixed together to meet a person's special needs. They're typically offered by medical spas, wellness and weight loss clinics, and pharmacies.
“Compounded drugs can be hugely beneficial for people who are allergic to certain ingredients in a commercial product, need a specific dose or dosage form that isn’t commercially available, or because of drug shortages,” Alyssa Billingsley, PharmD, director of pharmacy content for GoodRx, told Health. “In these cases, their doctor may choose to prescribe a compounded medication.”
Facilities that offer compounded drugs must follow state and federal regulations, which, among other things, require compounded drugs to be administered by a licensed healthcare provider and prohibit compounders from deceiving patients into believing products are FDA-approved or proven to achieve certain results.
However, as non-FDA-approved products, these medications have not been evaluated by the agency for safety or quality.
“Compounded drugs do not have the same safety, quality, and effectiveness assurances as FDA-approved drugs,” Priya Jaisinghani, MD, an endocrinologist and obesity medicine specialist and clinical assistant professor at NYU Langone Health, told Health.
The FDA said that overdoses stem from various issues, such as compounded semaglutide products often having packaging and dosing requirements that differ from FDA-approved products.
In some cases, healthcare providers incorrectly calculate the intended dose when converting from milligrams, which is what FDA-approved drugs use, to milliliters. The agency cited one provider who prescribed 20 units instead of 2 units, leading three patients to take 10 times the intended dose.
Other times, patients are simply confused about how much they should take. One patient who turned to the Internet for help with dosage instructions eventually took five times the intended dose, the agency reported.
“The problem seems to be that people aren’t being properly educated on how to accurately measure their dose from the vial, so they’re injecting a much larger amount in error,” Billingsley said.
Another issue with the compounded weight management drugs is that they don’t always contain the same active ingredient as FDA-approved versions. In May, the FDA issued a warning saying that some compounded semaglutide products contain salt formulations, such as semaglutide sodium and semaglutide acetate, that differ from the active ingredient in FDA-approved semaglutide. Those formulations haven't been shown to be safe or effective, the agency said.
“Poor compounding practices also can result in serious drug quality problems, including contamination,” Jaisinghani added. “There can also be differences in strength, quality, and purity.”
When it comes to using compounded semaglutide, Billingsley said that you should only do so when medically necessary and if your doctor has advised it.
“It’s also important to understand the risks,” she said. “Compounded semaglutide always requires a prescription, and your provider should tell you if you are being prescribed a compound.”
If you receive a vial with syringes, sublingual drops, nasal spray, or dissolvable tablets, there’s a good chance it’s compounded—and it’s important to ensure that you know how to use the product properly. While most pharmacies will have a pharmacist walk you through the process, it's a good idea to ask for one if that doesn’t happen.
After taking a compounded semaglutide, be on the lookout for side effects such as nausea, vomiting, or abdominal pain. If you suspect that symptoms are linked to the use of a compounded product, experts advise contacting a healthcare provider right away.




