Possible dispensability of AgRP neurons in the hypothalamus for body weight regulation
July 29, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
- fact-checked
- peer-reviewed publication
- trusted source
- proofread
by Ingrid Fadelli , Medical Xpress
Obesity has been linked to numerous health conditions, including high blood pressure, high cholesterol, type 2 diabetes, heart disease and strokes. Identifying neural processes underpinning excessive eating and obesity could thus have notable implications for global health, as it could help to devise targeted treatments that promote a healthy food intake.
Researchers at University of Texas Health Science Center at Houston and University of Science and Technology of China (USTC) recently carried out a study investigating the role of AgRP (agouti-related protein-expressing) neurons, a class of cells in the hypothalamus, in the maintenance of a healthy body weight. Their findings, published in Cell Reports, suggest that these neurons may not be as indispensable to the maintenance of body weight as previous works had suggested.
'Feeding is the primary way for animals and humans to acquire energy and nutrients,' Dr. Cheng Zhan, one of the researchers who carried out the study, told Medical Xpress. 'The amount of food consumed has a significant impact on overall health, including body weight, metabolism, immune function, and even lifespan. Our recent paper in Cell Reports came about through a series of research efforts that aimed to understand the mechanisms underlying weight maintenance and feeding regulation.'
Past research repeatedly highlighted the key role of the brain in regulating feeding and metabolism, both in humans and other animals. Many studies specifically focused on AgRP neurons in the hypothalamus (i.e., a small region at the center of the brain), as these neurons were found to increase food intake behavior in adult mice.
In 2011, researchers at Harvard Medical School used optogenetics and chemogenetics techniques to activate AgRP neurons in the mice brain, subsequently observing their behavior. They found that the activation of these neurons led to binge eating behaviors. Similarly, a 2020 paper published in Nature Metabolism showed that a prolonged enhanced activity of AgRP neurons resulted in mice becoming extremely obese. These studies demonstrate a sufficiency for AgRP neurons in promoting feeding and body weight gain.
'In 2005, researchers achieved selective expression of the human receptor for diphtheria toxin (DTR) on AgRP neurons in adult mice and injected diphtheria toxin,' Dr. Zhan explained. 'This led to the death of AgRP neurons within a few days, causing the mice to stop feeding, rapidly lose weight, and even starve to death.
'These findings have been widely cited and reiterated and have found their way into textbooks. For a long time, it has thus been widely believed that AgRP neurons in the hypothalamus are crucial and indispensable for feeding behavior and regulation. Our present study challenges the longstanding dogma that AgRP neurons are required for normal feeding and body weight regulation.'
This recent study was carried out in two different labs, one in the United States and the other in China. The first series of experiments was conducted by Dr. Tong's Lab at University of Texas Health Science Center of Houston.
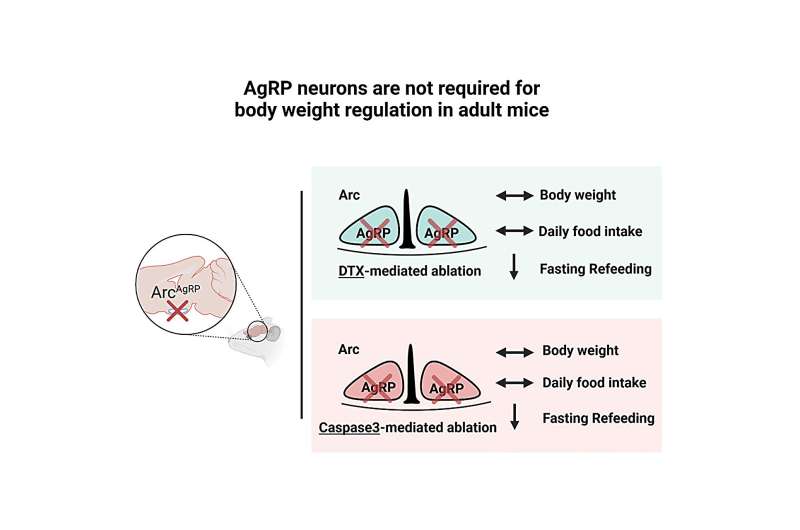
In these experiments, the researchers selectively expressed the human diphtheria toxin receptor on AgRP neurons in the brain of adult mice and injected low doses of diphtheria into their brain ventricles. Diphtheria is a toxic substance secreted by certain bacteria that inhibits the synthesis of proteins and can kill cells.
'Selectively expressing and injecting diphtheria was sufficient to kill AgRP neurons, but it did not affect the weight and basal feeding levels of the mice,' Dr. Zhan said. 'However, injecting high doses of diphtheria toxin not only caused weight and feeding reduction in diphtheria toxin receptor mice, but also in wild-type mice, and could even lead to death. These results suggest that the feeding and weight reduction observed in previous studies that killed AgRP neurons may be attributed to the non-specific toxicity of diphtheria toxin.'
In a separate set of experiments carried out by Dr. Zhan's Lab at USTC in China, the researchers tried to kill AgRP neurons using a different method, to assess the impact of this on the mice's feeding behavior. Specifically, they selectively expressed the Caspase-3 gene, inducing the apoptosis (i.e., programmed death) of AgRP,
'We found that killing AgRP neurons using this method did not decrease the weight and feeding of adult mice,' Dr. Zhan said. 'Interestingly, although killing AgRP neurons did not affect basal feeding and weight, it had some impact on refeeding after fasting, indicating that AgRP neurons may play a role in responding to environmental stress. Using two different methods to kill AgRP neurons, our study concluded that AgRP neurons are not essential for maintaining basal feeding and weight under laboratory conditions but may be more important for feeding and weight regulation under conditions of food scarcity.'
The recent findings gathered by Dr. Zhan, Dr. Tong and their colleagues challenge the common understanding of AgRP neurons as indispensable for feeding behavior and body weight maintenance. In addition, their work suggests that feeding behavior is regulated by complex neural mechanisms involving multiple brain nuclei and neuronal subgroups. In the future, this study could pave the way for further research exploring the role of AgRP neurons and other neuron populations in regulating feeding behavior.
'In recent years, research has discovered several brain regions that possess feeding-promoting functions,' Dr. Zhan added. 'As a next step, we will focus on studying the robustness of feeding behavior and the redundancy in its regulatory mechanisms. This includes investigating the interconnections between different orexigenic neurons and exploring new targets for feeding regulation.
'Further research will provide a comprehensive understanding of the neural mechanisms underlying feeding regulation and offer new targets and insights for controlling diet and treating eating disorders.'
© 2023 Science X Network




