Hybrid Photocatalysts for Water Splitting with Internal Quantum Efficiency Over 100%
May 5, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
- fact-checked
- peer-reviewed publication
- trusted source
- proofread
by Ingrid Fadelli, Tech Xplore
As hydrogen inside fuel cells can generate electrical power, scalable methods to reliably split water into hydrogen and oxygen could have valuable implications for the energy industry. These methods could help to produce large amounts of hydrogen for more sustainable energy solutions, helping to reduce greenhouse gas emissions on Earth.
One approach to split water molecules into hydrogen and oxygen requires the use of photocatalysts, materials that can absorb light and use its energy to initiate chemical reactions. This approach essentially entails irradiating these materials with light, triggering the reaction through which water molecules become hydrogen and oxygen.
Researchers at Northwestern Polytechnical University in China recently introduced new hybrid photocatalysts that exhibit a remarkable internal quantum efficiency above 100%. These materials, introduced in a paper in Nature Energy, were found to overcome some of the shortcomings of previously proposed photocatalytic systems for water splitting processes.
'Over the past decade, researchers have made numerous attempts to achieve a solar-to-hydrogen efficiency of more than 10%, which is a competitive benchmark efficiency in the hydrogen market,' Dr. Xuanhua Li, one of the researchers who carried out the study, told Tech Xplore.
'To achieve this goal, the internal quantum efficiency (the ratio of the number of incident photons absorbed to twice the amount of hydrogen produced) of the photocatalyst during the photocatalytic water splitting reaction must reach a moderately high value (ideally >100%) over a wide range of excitation wavelengths.'
Several past studies have tried to devise useful strategies to improve the quantum efficiency of photocatalysts and photoelectric devices, as most previously reported efficiencies were insufficient to enable the widespread use of water splitting processes. One strategy that was found to be particularly promising leverages the so-called multiple exciton generation (MEG) effect, in which a nanocrystal quantum dot absorbs a single photon to generate multiple excitons.
'For example, one study showed that the quantum efficiency of lead-salt nanocrystals increases roughly linearly with pump-photon energy and exhibits a maximum quantum efficiency up to 700%,' Dr. Li explained. 'As shown in previous works, depositing the PbS quantum dots on the top of fluorine-doped tin oxide/TiO2 via a layer-by-layer approach can achieve an IQE that exceeds 100% in photoelectrochemical cells for hydrogen generation. Compared with photoelectric devices, however, demonstrations of the MEG effect are still scarce in particulate photocatalytic water splitting system due to the addition process for electric energy to hydrogen energy.'
The key objective of the recent work by Dr. Li and his colleagues was to design new photocatalysts for efficient water splitting that utilize the MEG effect. Their hope was that the internal quantum efficiency of these materials would exceed 100%, making them a viable solution for the scalable production of hydrogen.
To construct these efficient photocatalysts, the researchers had to construct a strong interfacial built-in electric field and an interfacial trapping state. This would in turn provide a sufficient driving force for them to use the multiple exciton generation (MEG) effect in photocatalytic water splitting.
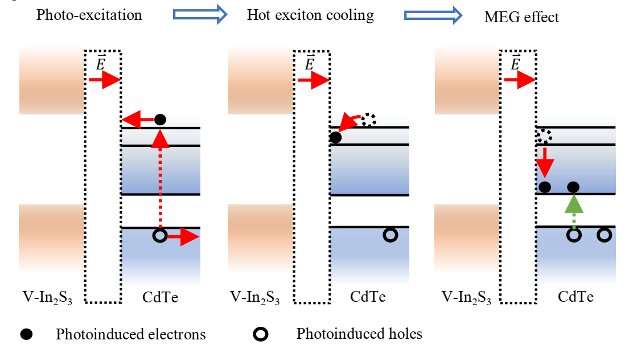
'We developed hybrid photocatalysts comprising CdTe quantum dots and V-doped In2S3 (CdTe/V-In2S3),' Dr. Li said. 'Specifically, increasing the quantum dot size and V-dopant content leads to a downshift in the Fermi level of the CdTe quantum dots and an upshift in that of V-In2S3, resulting in an increase in the Fermi level difference and thus a 14.14 folds increase in the built-in electric field intensity at the CdTe/V-In2S3 interface. Meanwhile, an interfacial state composed of In 5s and S 3p orbitals at CdTe/V-In2S3 interface generated.'
The excitation of a CdTe quantum dot in the team's photocatalyst during the photocatalytic process results in the generation of a hot electron and a hole. Driven by the materials' built-in electric field, the hot electron in the conduction band of the CdTe quantum dot is transported from CdTe to V-In2S3 and ultimately trapped at the CdTe/V-In2S3 interface, in an interfacial state made up of In 5s and S 3p orbitals.
'Unlike traditional photocatalyst, the strong built-in electric field and interfacial state at the CdTe/V-In2S3 interface slow the relaxation rate of the hot electrons, enabling hot electrons with sufficient excess energy to undergo MEG,' Dr. Li said. 'Ultimately, the photocatalyst exhibits an internal quantum efficiency of about 114% at an excitation wavelength of 350 nm, which to our knowledge is the highest value among reported photocatalysts for overall water splitting. Our optimization of the interfacial built-in electric field and interfacial state, paves a way for the effective utilization of MEG in photocatalytic water splitting.'
In initial evaluations, the hybrid photocatalysts designed by this team of researchers achieved very promising results, exhibiting higher internal quantum efficiencies than all previously proposed photocatalysts for water splitting. In the future, this recent work could pave the way for the large-scale implementation of photocatalytic water splitting.
The design strategy presented by Dr. Li and his colleagues also opens new possibilities for the design of photocatalytic devices that operate in the MEG regime. This could soon lead to the development of additional materials and solutions with increasingly high quantum and solar-to-hydrogen efficiencies, which could further promote the use of solar energy to produce hydrogen.
'It should be noted that the photocatalytic overall water splitting ability of CdTe/V-In2S3 might be limited by the competition for light absorption between V-In2S3 and the CdTe quantum dots,' Dr. Li added. 'Moreover, achieving high quantum efficiency over a wide range of wavelengths is crucial to1advancing the practicality of this technology. To further advance this research, we aim to develop more efficient photocatalysts with larger built-in electric field intensities and multiple valence band interfacial states, such as by constructing a Janus structure.'
In their next studies, Dr. Li and his colleagues also plan to create new non-MEG/MEG heterojunctions with a broad absorption range. By combining wavelength-complementary MEG components with non-MEG components, they hope to further improve the photocatalysts' overall performance.
© 2023 Science X Network




