"Search for Room-Temperature Superconductivity Made Easier with New Material"
May 12, 2023
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
- fact-checked
- peer-reviewed publication
- trusted source
- proofread
by Skolkovo Institute of Science and Technology
Scientists from Jilin University, the Center for High Pressure Science and Technology Advanced Research, and Skoltech have synthesized lanthanum-cerium polyhydride, a material that promises to facilitate studies of near-room-temperature superconductivity. It offers a compromise between the polyhydrides of lanthanum and cerium in terms of how much cooling and pressure it requires. This enables easier experiments, which might one day lead scientists to compounds that conduct electricity with zero resistance at ambient conditions—an engineering dream many years in the making. The study was published in Nature Communications.
One of the most intriguing unsolved questions in modern physics is: Can we make a material that conducts electricity with zero resistance (superconducts) at room temperature and atmospheric pressure? Such a superconductor would enable power grids with unprecedented efficiency, ultrafast microchips, and electromagnets so powerful they could levitate trains or control fusion reactors.
In their search, scientists are probing multiple classes of materials, slowly nudging up the temperature they superconduct at and decreasing the pressure they require to remain stable. One such group of materials is polyhydrides—compounds with extremely high hydrogen content. At -23°C, the current champion for high-temperature superconductivity is a lanthanum polyhydride with the formula LaH10. The trade-off: It requires the pressure of 1.5 million atmospheres. At the opposite end of the spectrum, cuprates are a class of materials that superconduct under normal atmospheric pressure but require cooler temperatures—no more than -140°.
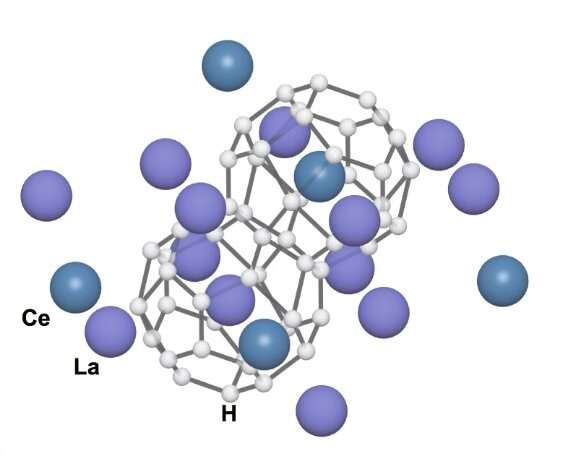
Now, Skoltech researchers and their Chinese colleagues have managed to loosen up the pressure requirements of polyhydride superconductors. To do this, the team tweaked the lanthanum-hydrogen system by adding some cerium into the mix. Physically, that meant making a lanthanum-cerium alloy and heating it up in a high-pressure cell with ammonia borane, a substance that releases a lot of hydrogen as it decomposes.
Lanthanum and cerium are two very similar atoms that form analogous compounds and may often be substituted for one another. However, while superconductivity has been reported for the polyhydrides LaH10 and CeH10, as well as CeH9, the corresponding LaH9 phase is rarely seen by experimenters. The scientists decided to test out the hypothesis: It should be possible to stabilize LaH9 by supplementing it with an appropriately chosen additive, such as cerium, provided that this alters the original material's structure. And it worked.
'Very high pressure forces pure lanthanum and hydrogen into the LaH10 structure. But if you replace roughly 1 in 4 lanthanum atoms with cerium, this reorganizes the structure into the arrangement seen in CeH9. In that sense, the introduction of the third element alters the structure that the pure material would have otherwise assumed. And this additive does contribute to stability: Compared with the 1.5 million atmospheres you need for LaH10, our lanthanum-cerium polyhydride is stable at just 1 million atmospheres. This is about the same pressure the cerium polyhydrides require, yet those exhibit superconductivity only below -158°C, whereas the new superconductor works at -97°C. So it's a good compromise, but more importantly, it's a reassurance that our reasoning is right,' study co-author Professor Artem R. Oganov of Skoltech comments.
Working in a field where not too long ago, many doubted that so-called conventional superconductivity—like the one in polyhydrides—could ever exist at temperatures above -230°C or so, Oganov assigns particular importance to testing out and refining the rules that make it possible to discover and improve superconductors in a reliable and systematic way. So even though he thinks that polyhydrides in general will hardly ever be made to superconduct at atmospheric pressure (which is a necessary condition for large-scale applications such as maglev trains or lossless power grids), he says their study affords insights into superconductivity that take us closer to attaining that ultimate goal with other materials.
'Polyhydrides are an Eldorado for fundamental superconductor research under pressure,' Oganov says. 'And by synthesizing our new compound, we have both tested and refined the tools and tricks useful in this quest and supplied a convenient material for further studies.'
'The work is also interesting for two key experiments: It shows the possible anisotropy of the upper critical field for hydrides. That is, the dependence of critical temperature on the direction of the magnetic field. And it also shows that with a decrease in pressure, a pseudogap phase manifests in polyhydrides,' study co-author and Skoltech Ph.D. alumnus Dmitrii Semenok said, adding that both properties are characteristic of cuprate superconductors. 'Thus, on closer inspection, polyhydrides turn out to be much like cuprates despite the different mechanisms of superconductivity.'
Asked about other promising compounds that current polyhydride research is leading to, the researchers responded that hydrides and borohydrides of calcium, yttrium, lanthanum, and magnesium seem to deserve research attention at this point.
Journal information: Nature Communications
Provided by Skolkovo Institute of Science and Technology




