Newly Discovered Nanorings: Groundbreaking Ingredients for Chemistry
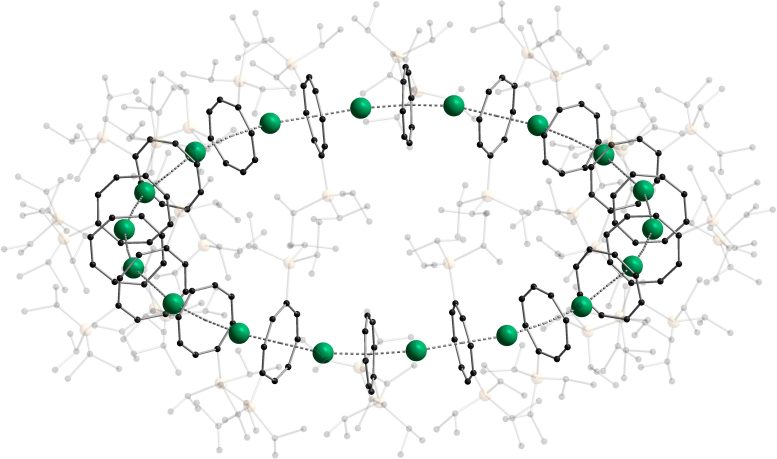
The recent discovery of a new molecular structure called ‘cyclocene,' where sandwich complexes form a nanoscale ring, has been creditted to Nature/AOC, KIT.
The cutting-edge compounds for Organometallic Chemistry consist of sandwich complexes that form rings and maintain their own energy.
Sandwich compounds are used as primary building blocks in organometallic chemistry because of their unique and specialised chemical composition. These structures were linear until recently when scholars from Karlsruhe Institute of Technology (KIT) and the University of Marburg created a nanoscale ring from sandwich compounds stacked together. Researchers will now conduct further studies on the physical properties and other aspects of these cyclocene structures.
Two parallel-layered flat aromatic organic rings, similar to slices of a sandwich, with a single metal atom in between, form the structure of sandwich complexes developed about seven decades ago. Additional layers of the ‘bread’ and the ‘filling’ can produce multiple sandwich complexes.
According to Professor Peter Roesky of KIT's Institute for Inorganic Chemistry, these compounds are vital to modern organometallic chemistry.
Ferrocene, a highly durable compound formed by an iron ion sandwiched between two five-membered aromatic organic rings, was awarded the Nobel Prize in Chemistry in 1973. It is widely used in synthesis, catalysis, electrochemistry, and polymer chemistry.
Researchers at KIT and the University of Marburg have long aimed to organise the sandwich complexes into a ring form. While they initially only produced chains, the collaboration between the three teams at the two universities has now resulted in nanoscale rings.
Professor Manfred Kappes, head of KIT's Division of Physical Chemistry of Microscopic Systems, and Professor Florian Weigend, Head of the Applied Quantum Chemistry Unit of the University of Marburg, report the successful formation of nanoscale rings for the first time due to the appropriate selection of organic intermediate layers or 'slices of bread.'
The new nanoring, comprised of 18 building blocks and with a diameter of 3.8 nanometers, yields an orange-coloured photoluminescence depending on the metal used as the ‘filling’ of the sandwich. The scientists have termed this new chemical compound as 'cyclocene.'
The three teams utilised detailed quantum chemical calculations to examine why the molecules could be organised into a ring instead of forming a customary chain of sandwich complexes. This investigation revealed that the energy derived from closing the ring was the primary driving force behind its formation.
“Initially, the aim was simply to form a ring. The exploration of other ring sizes and identifying any unusual physical properties that this nanostructure might possess is the target of future research. But as of now, it's clear that we've expanded our toolbox of organometallic chemistry with a new addition, and this is a significant achievement,” says Roesky.




