Insights into Future Biofuels: Leafcutter Ants Cultivate Fungal Gardens to Degrade Plants
1st February 2024
The article you’re about to read has been deeply scrutinized according to the editorial process and policies of Science X. To gain confidence in our content, our editors have capitalized on the following definitive features:
- facts verification
- trusted references
- proofreading
reviewed by Maegan Murray, Pacific Northwest National Laboratory
Over the decades, scientists have made impressive strides in the efficient and cost-effective degradation of plant substances into valuable bioproducts that make a difference in our everyday lives.
From bio-based fuels, detergents, nutritional supplements, to plastics, scientific innovations have made it possible to degrade plants suitable for manufacturing a plethora of products. However, certain polymers such as lignin, found in the plant cell wall, pose a challenge. They're hard to break down cost-effectively without contributing pollutants back to the environment. These polymers often end up as waste materials with no further utility.
There is a known specialized microbial community made up of fungus, leafcutter ants, and bacteria. This community naturally decomposes plants into nutrients and other components that are absorbed and utilised by surrounding organisms and systems. However, unearthing all the components and biochemical reactions necessary for this process has been a considerable challenge—until now.
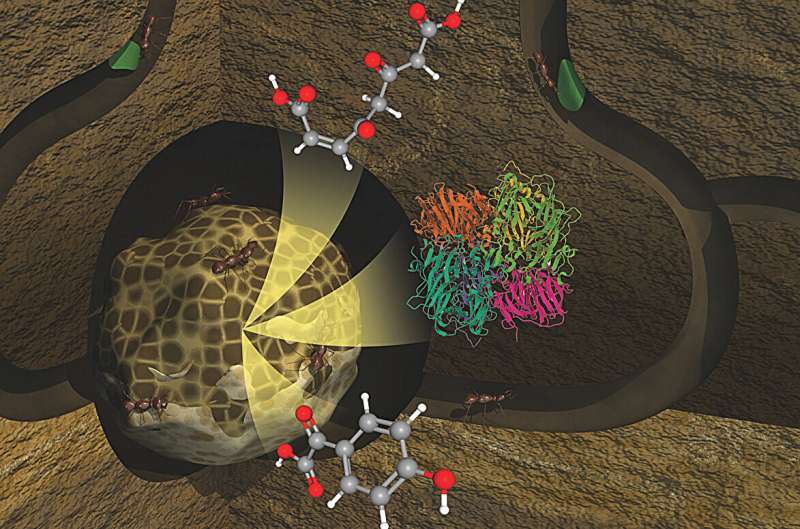
Under the guidance of Kristin Burnum-Johnson, science group leader for Functional and Systems Biology at Pacific Northwest National Laboratory (PNNL), a team of fellow researchers at PNNL have designed a revolutionary imaging technique called metabolome informed proteome imaging (MIPI). This technique permits scientists to delve deep into the molecular level and visualize the components involved in the plant degradation process, and explore the who, what, when, and where of significant biochemical reactions.
By deploying this technique, the team successfully identified essential metabolites and enzymes that trigger various biochemical reactions significant in the degradation procedure. They also discovered the role of resident bacteria in the system contributing to improve efficiency. These findings can be instrumental in the research and development of future biofuels and bioproducts.
Their research is now featured in the prestigious Nature Chemical Biology.
The key to successful plant degradation lies in the symbiotic relationship between leafcutter ants and fungus
The team focused their research on a fungus known as Leucoagaricus gongylophorus, that is recognized for its symbiotic relationship with a species of leafcutter ants. The ants employ this fungal strain to maintain a fungal garden that degrades plant polymers and other substances. The leftover components from the degradation process are consumed by other organisms in the garden, allowing all inhabitants to thrive.
This process is facilitated by the ants cultivating the fungus on fresh leaves in distinctive underground structures. These structures eventually transit into the bountiful fungal gardens. Resident bacteria present in the circuit contribute to the degradation by producing amino acids and vitamins which augment the entire garden ecosystem.
According to Burnum-Johnson, 'Environmental systems have evolved over millions of years into impeccable symbiotic systems. What better way to learn and benefit from these systems than by observing their natural operations?'
The intense degree of complexity contained in these fungal communities is what makes them challenging to study. While the plants, fungus, ants, and bacteria all play vital roles in the plant degradation process, none focuses solely on one task or resides exclusively in one location. With vital biochemical reactions taking place at the molecular level, deciphering this becomes a complex puzzle. However, the newly developed MIPI imaging method at PNNL is unveiling the intricate scenarios of degradation process.
'For the first time, we now have the necessary tools to completely comprehend the complexities of these systems, and also visualize them as an integrated unit', affirmed Burnum-Johnson. Using a powerful laser, the team captured scans across 12-micron-thick sections of a fungal garden, which is almost the same width as a sheet of plastic kitchen wrap. This enabled the identification of metabolite locations and detection of the site and abundance of plant polymers such as cellulose, xylan, and lignin as well as other molecules. This led them to "hot spots" or concentrated areas where plant matter had been degraded.
Focusing on these regions, they were able to distinguish enzymes, the catalysts for biochemical reactions in living systems. Understanding the type and location of these enzymes helped them identify which microbes were actively engaged in the degradation process.
All of these components together helped affirm the fungus as the primary degrader of the plant material in the system. Additionally, the team determined that the bacteria present in the system transformed previously digested plant polymers into metabolites that are used as vitamins and amino acids in the system. These vitamins and amino acids benefit the entire ecosystem by accelerating fungal growth and plant degradation.
Burnum-Johnson said if scientists had used other more traditional methods that take bulk measurements of primary components in a system, such as metabolites, enzymes, and other molecules, they would simply get an average of those materials, creating more noise and masking information.
'It dilutes the important chemical reactions of interest, often making these processes undetectable,' she said. 'To analyze the complex environmental ecosystems of these fungal communities, we need to know those fine detail interactions. These conclusions can then be taken back into a lab setting and used to create biofuels and bioproducts that are important in our everyday life.'
Marija Velickovic, a chemist and lead author of the paper, said she initially became interested in studying the fungal garden and how it degrades lignin based on the difficulty of the project.
'Fungal gardens are the most interesting because they are one of the most complex ecosystems composed of multiple members that effectively work together,' she said. 'I really wanted to map activities at the microscale level to better understand the role of each member in this complex ecosystem.'
Velickovic performed all the hands-on experiments in the lab, collecting material for the slides, scanning the samples to view and identify metabolites in each of the sections, and identifying hot spots of lignocellulose degradation.
Both Velickovic and Burnum-Johnson said they are ecstatic about their team's success.
'We actually accomplished what we set out for,' Burnum-Johnson said. 'Especially in science, that isn't guaranteed.'
The team plans to use its findings for further research, with specific plans to study how fungal communities respond and protect themselves amid disturbances and other perturbations.
'We now have an understanding of how these natural systems degrade plant material very well,' Burnum-Johnson said. 'By looking at complex environmental systems at this level, we can understand how they are performing that activity and capitalize on it to make biofuels and bioproducts.'
Provided by Pacific Northwest National Laboratory




