Development of a Expandable Photoelectrochemical System for Environmentally Friendly Hydrogen Generation
February 10, 2024
feature
This article has passed the editorial process and policies of Science X and has the following credibility markers:
- It is fact-checked
- It has been peer-reviewed
- It is sourced from a trusted source
- It is proofread
Author: Ingrid Fadelli, Tech Xplore
Solar energy is being explored as a potential method to generate hydrogen gas (H2) on a large scale using water splitting. However, current photochemical water splitting methods are either inconsistent, unstable or challenging to implement on a large scale.
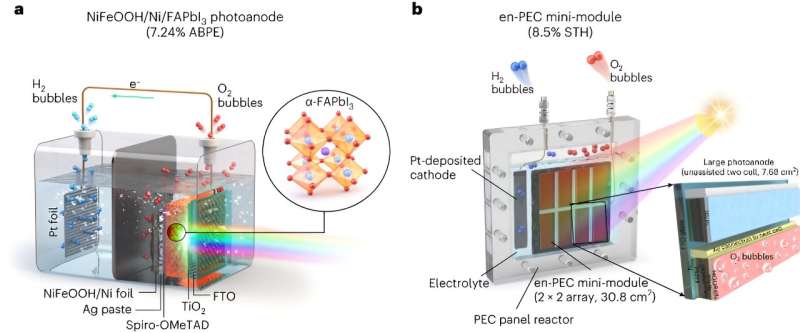
Scientists at Ulsan National Institute of Science and Technology (UNIST) are working towards creating an efficient photoelectrochemical (PEC) method for green hydrogen production. They proposed a system that uses formamidinium lead triiodide (FAPbI3) perovskite-based photoanode, safeguarded by an Ni foil/NiFeOOH electrocatalyst.
Jae Sung Lee, a Professor of Energy & Chemical Engineering at UNIST, emphasized the need for at least a 10% solar-to-hydrogen (STH) efficiency for effective practical PEC systems which require the selection of an optimal material.
Previous efforts in PEC hydrogen production primarily involved the use of metal oxides which are inherently stable. However, these systems have fallen short in achieving the necessary efficiency levels for practical applications. Therefore, some researchers have begun to study the potential of photovoltaic (PV) grade materials which are known for remarkable efficiency but can be expensive and unstable in water.
According to Lee, metal-halide perovskites (MHP) could be an alternative for photoelectrode materials due to their high efficiency and low cost. The paramount challenge, however, is to ensure their stability in humid conditions and under UV light which led the researchers to explore UV-stable FAPbI3 perovskite.
Lee explains the importance of achieving scalability while maintaining high efficiency for practical applications. The lead candidate MHP material to achieve this was FAPbI3. It was encapsulated with a thick nickel foil and NiFeOOH catalyst to enable an oxygen evolution reaction for water splitting.
On the laboratory scale, their device achieved 9.89% STH efficiency and maintained stability. This was subsequently scaled to a larger size using a module-based design with minimal loss of efficiency and stability.
Lee summarized that their entire PEC system comprised a FAPbI3 photoanode which used MHP thin film protected by a nickel metal foil and a NiFeOOH catalyst layer on top of it.
'We optimized this photoanode using different metal foils and studied the in-depth catalyst-electrolyte interactions. This photoanode was connected in parallel to another MHP thin film as PV in a single reactor to generate enough voltage (~2 V) to split water molecules into O2 and H2 gases. In a large scale system, both components (photoanode and PV) are integrated in single PEC device to simplify the total system by a modular design.'
The researchers' mini-module is essentially made up of a photoelectrode and a PV unit cell, arranged in a 4 x 4 array. Their system integrates multiple components in a single PEC device to eliminate the need for additional PV components.
This unique design reduces their system's complexity and lowers its fabrication costs. Lee and his colleagues demonstrated that their system retains a good performance even when deployed in a larger scale, which could facilitate its future real-world deployment.
'The short-term demonstration of our scalable system will lead toward practical application of PEC technology for green hydrogen production in outdoor conditions,' Lee added. 'We also plan to further improve the efficiency and stability of the PEC system by integration of photoelectrodes and selecting more efficient and durable catalyst. We look [forward to] the opportunity to demonstrate a pilot scale solar hydrogen production system under natural sunlight using our technology.'
© 2024 Science X Network




