"Transformative Advances in H2O2 Production: Breakthrough Potential of Ultrathin Nanosheets"
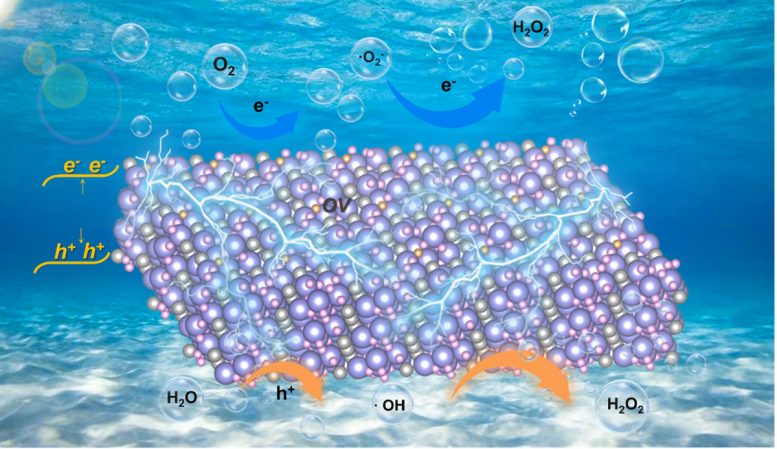
Bi4O5Br2 is highly attractive as an efficient piezocatalyst that utilizes the ubiquitous mechanical energy for H2O2 synthesis. Oxygen vacancies mediated ultrathin Bi4O5Br2 nanosheets display a better piezoelectric response and stronger adsorption and activation ability of oxygen, leading to an outstanding piezocatalytic H2O2 synthesis performance without any sacrificial agents and co-catalysts in pure water. Credit: Chinese Journal of Catalysis
Recent research has demonstrated the effectiveness of ultrathin Bi4O5Br2 nanosheets with controlled oxygen vacancies in enhancing the piezocatalytic production of hydrogen peroxide (H2O2), presenting a viable, environmentally friendly alternative to traditional methods.
Hydrogen peroxide (H2O2) serves as a crucial chemical raw material with extensive applications in numerous industrial and everyday contexts. However, the industrial anthraquinone method of producing H2O2 is fraught with significant drawbacks, including high levels of pollution and energy consumption. An alternative approach involves harnessing ubiquitous mechanical energy for piezocatalytic H2O2 evolution, which offers a promising strategy. Despite its potential, this method faces challenges due to its unsatisfactory energy conversion efficiency.
Bi4O5Br2 is regarded as a highly attractive photocatalytic material due to its unique sandwich structure, excellent chemical stability, good visible light capture ability, and suitable band structure. Aspired by its non-centrosymmetric crystal structure, piezoelectric performance has begun to enter the vision of researchers recently. However, its potential as an efficient piezocatalyst is far from being exploited, especially since the impacts of defects on piezocatalysis and piezocatalytic H2O2 production over Bi4O5Br2 remains scanty. Thus, mechanical energy-driven piezocatalysis provides a promising method for H2O2 synthesis from pure water with great attraction.
Recently, a research group led by Prof. Hongwei Huang from China University of Geosciences reported outstanding piezocatalytic H2O2 evolution performance that was achieved over ultrathin Bi4O5Br2 nanosheets with appropriate oxygen vacancies, and disclosed the mechanism that thin structure and oxygen vacancies collectively enhance the piezocatalytic activity. The results were published in the Chinese Journal of Catalysis.
Ultrathin Bi4O5Br2 nanosheets with controllable oxygen vacancy concentrations are synthesized by a one-step solvothermal method by tuning the water to ethylene glycol ratio. Experiments and theoretical calculations have shown that Bi4O5Br2 with appropriate oxygen vacancies exhibits dramatic performance for piezocatalytic H2O2 production. On the one hand, oxygen vacancies and thin structure largely increase the piezoelectric properties and piezoelectric potential of Bi4O5Br2, which improve the separation and transfer of piezoinduced charges. On the other hand, oxygen vacancies promote oxygen adsorption and activation on the surface of Bi4O5Br2, and lead to constantly decreased Gibbs free energy of the reaction pathway. Therefore, the piezocatalytic H2O2 production performance of Bi4O5Br2 with appropriate oxygen vacancies is higher than that of other commonly used piezocatalysts.




